Singapore's Respiree Secures FDA Clearance for Home Health Tech Innovation
Singapore-based Respiree secures second FDA clearance for its innovative cardio-respiratory wearable device, expanding into home healthcare monitoring with AI-powered solutions.
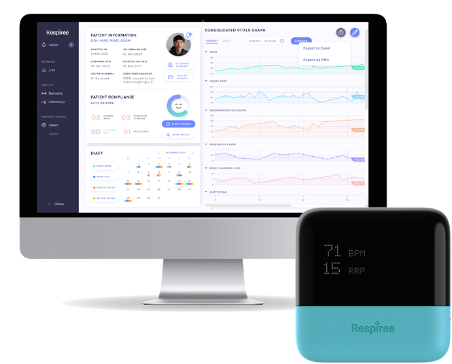
Respiree's RS001 wearable device and 1Bio™ connected care platform showcasing Singapore's healthcare innovation
Singapore-based Respiree Expands Healthcare Innovation with FDA Approval
Singapore health tech company Respiree has achieved a significant milestone with its second FDA 510(k) clearance, extending the use of its RS001 cardio-respiratory wearable device to home environments. This advancement, announced on August 5, 2025, includes approval for the company's 1Bio™ connected care platform, marking a notable achievement in Singapore's growing influence in healthcare technology innovation.
Revolutionary Home Health Monitoring
The RS001, a chest-worn device, provides direct respiratory measurements and passive cardio-respiratory monitoring. This expansion to home settings represents a significant step in preventive healthcare, particularly as Singapore continues to lead digital transformation initiatives in healthcare delivery.
"Respiration is the most predictive vital sign for clinical deterioration, yet it remains underutilized - particularly outside hospital settings," explains Dr. Gurpreet Singh, CEO and Founder of Respiree.
Strategic Growth and Investment
Following a successful US$11.6 million Series A financing round led by We Venture Capital and ClavystBio, Respiree demonstrates Singapore's growing capability in attracting significant international investment for healthcare innovation.
Key Features of the 1Bio™ Platform:
- Real-time data visualization through cellular hub connectivity
- Passive monitoring capabilities for early intervention
- Integration potential for AI-driven healthcare solutions
- Cross-border healthcare management capabilities
The company's focus on AI integration positions it at the forefront of healthcare technology innovation, with plans to pursue additional FDA clearances for its proprietary AI software as a Software as Medical Device (SaMD).
Wei-Ling Tan
Tech and economy specialist, covering innovation in Southeast Asia from Singapore for both English-language and regional media outlets.
